Abstract
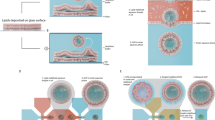
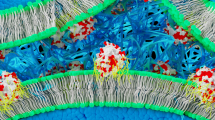
The design and construction of synthetic therapeutic protocells capable of establishing cognate chemical communication channels with living cells is an important challenge for synthetic biology and bio-engineering. Here we develop a step towards protocell-mediated nitric-oxide-induced vasodilation by constructing a new synthetic cell model based on bio-derived coacervate vesicles with high haemocompatibility and increased blood circulation times. The hybrid protocells are prepared by the spontaneous self-assembly of haemoglobin-containing erythrocyte membrane fragments on the surface of preformed polysaccharide–polynucleotide coacervate micro-droplets containing glucose oxidase. We use the sequestered enzymes to program a spatially coupled glucose oxidase/haemoglobin reaction cascade, which in the presence of glucose and hydroxyurea generates a protocell-mediated flux of nitric oxide that we exploit for in vitro and in vivo blood vessel vasodilation. Taken together, our results provide new opportunities for the development of endogenously organized cell-like entities (biocompatible micro-bots) geared specifically towards active interfacing with individual living cells and cell communities.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data analysed and used to support this study can be found within the main text and Supplementary Information.
References
Mage, P. L. et al. Shape-based separation of synthetic microparticles. Nat. Mater. 18, 82–89 (2019).
Brenner, J. S. et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 9, 2684 (2018).
Sercombe, L. et al. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 6, 286 (2015).
Monteiro, N., Martins, A., Reis, R. L. & Neves, N. M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 11, 20140459 (2014).
Zylberberg, C., Gaskill, K., Pasley, S. & Matosevic, S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 24, 441–452 (2017).
Petersen, A. L., Hansen, A. E., Gabizon, A. & Andresen, T. L. Liposome imaging agents in personalized medicine. Adv. Drug Deliv. Rev. 64, 1417–1435 (2012).
Rikken, R. S. M. et al. Shaping polymersomes into predictable morphologies via out-of-equilibrium self-assembly. Nat. Commun. 7, 12606 (2016).
Che, H., Cao, S. & van Hest, J. C. M. Feedback-induced temporal control of “breathing” polymersomes to create self-adaptive nanoreactors. J. Am. Chem. Soc. 140, 5356–5359 (2018).
Li, M., Harbron, R., Weaver, J., Binks, B. & Mann, S. Electrostatically gated membrane permeability in inorganic protocells. Nat. Chem. 5, 529–536 (2013).
Huang, X. et al. Interfacial assembly of protein–polymer nano-conjugates into stimulus-responsive biomimetic protocells. Nat. Commun. 4, 2239 (2013).
Huang, X., Patil, A. J., Li, M. & Mann, S. Design and construction of higher-order structure and function in proteinosome-based protocells. J. Am. Chem. Soc. 136, 9225–9234 (2014).
Kumar, B. V. V. S. P., Patil, A. J. & Mann, S. Enzyme-powered motility in buoyant organoclay/DNA protocells. Nat. Chem. 10, 1154–1163 (2018).
Jang, W.-S., Kim, H. J., Gao, C., Lee, D. & Hammer, D. A. Enzymatically powered surface-associated self-motile protocells. Small 14, 1801715 (2018).
Rodríguez-Arco, L., Li, M. & Mann, S. Phagocytosis-inspired behaviour in synthetic protocell communities of compartmentalized colloidal objects. Nat. Mater. 16, 857–863 (2017).
Qiao, Y., Li, M., Booth, R. & Mann, S. Predatory behaviour in synthetic protocell communities. Nat. Chem. 9, 110–119 (2017).
Joesaar, A. et al. DNA-based communication in populations of synthetic protocells. Nat. Nanotechnol. 14, 369–378 (2019).
Sun, S. et al. Chemical signaling and functional activation in colloidosome-based protocells. Small 12, 1920–1927 (2016).
Schwarz-Schilling, M., Aufinger, L., Mückl, A. & Simmel, F. C. Chemical communication between bacteria and cell-free gene expression systems within linear chains of emulsion droplets. Integr. Biol. 8, 564–570 (2016).
Adamala, K. P., Martin-Alarcon, D. A., Guthrie-Honea, K. R. & Boyden, E. S. Engineering genetic circuit interactions within and between synthetic minimal cells. Nat. Chem. 9, 431–439 (2017).
Tang, T.-Y. D. et al. Gene-mediated chemical communication in synthetic protocell communities. ACS Synth. Biol. 7, 339–346 (2018).
Gobbo, P. et al. Programmed assembly of synthetic protocells into thermoresponsive prototissues. Nat. Mater. 17, 1145–1153 (2018).
Koga, S., Williams, D. S., Perriman, A. W. & Mann, S. Peptide–nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 3, 720–724 (2011).
Merindol, R., Loescher, S., Samanta, A. & Walther, A. Pathway-controlled formation of mesostructured all-DNA colloids and superstructures. Nat. Nanotechnol. 13, 730–738 (2018).
Martin, N. et al. Antagonistic chemical coupling in self-reconfigurable host–guest protocells. Nat. Commun. 9, 3652 (2018).
Tian, L. et al. Nonequilibrium spatiotemporal sensing within acoustically patterned two-dimensional protocell arrays. ACS Cent. Sci. 4, 1551–1558 (2018).
Drobot, B. et al. Compartmentalised RNA catalysis in membrane-free coacervate protocells. Nat. Commun. 9, 3643 (2018).
Poudyal, R. R. et al. Template-directed RNA polymerization and enhanced ribozyme catalysis inside membraneless compartments formed by coacervates. Nat. Commun. 10, 490 (2019).
Zhang, Y., Baekgaard-Laursen, M. & Städler, B. Small subcompartmentalised microreactors as support for hepatocytes. Adv. Healthc. Mater. 6, 1601141 (2017).
Balasubramanian, V. et al. Biomimetic engineering using cancer cell membranes for designing compartmentalized nanoreactors with organelle‐like functions. Adv. Mater. 29, 1605375 (2017).
Godoy-Gallardo, M., Labay, C., Jansman, M. M. T., Ek, P. K. & Hosta-Rigau, L. Intracellular microreactors as artificial organelles to conduct multiple enzymatic reactions simultaneously. Adv. Healthc. Mater. 6, 1601190 (2017).
Thingholm, B., Schattling, P., Zhang, Y. & Städler, B. Subcompartmentalized nanoreactors as artificial organelle with intracellular activity. Small 12, 1806–1814 (2016).
Tanner, P., Balasubramanian, V. & Palivan, C. G. Aiding nature’s organelles: artificial peroxisomes play their role. Nano Lett. 13, 2875–2883 (2013).
Einfalt, T. et al. Biomimetic artificial organelles with in vitro and in vivo activity triggered by reduction in microenvironment. Nat. Commun. 9, 1127 (2018).
Gardner, P. M., Winzer, K. & Davis, B. G. Sugar synthesis in a protocellular model leads to a cell signalling response in bacteria. Nat. Chem. 1, 377–383 (2009).
Lentini, R. et al. Integrating artificial with natural cells to translate chemical messages that direct E. coli behaviour. Nat. Commun. 5, 4012 (2014).
Lentini, R. et al. Two-way chemical communication between artificial and natural cells. ACS Cent. Sci. 3, 117–123 (2017).
Xia, Y. et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 17, 187–194 (2018).
Zhou, Y. et al. In situ gelation-induced death of cancer cells based on proteinosomes. Biomacromolecules 188, 2446–2453 (2017).
Chen, Z. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 14, 86–93 (2018).
Crosby, J. et al. Stabilization and enhanced reactivity of actinorhodinpolyketide synthase minimal complex in polymer–nucleotide coacervate droplets. Chem. Commun. 48, 11832–11834 (2012).
Sokolova, E. et al. Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate. Proc. Natl Acad. Sci. USA 110, 11692–11697 (2013).
Tang, T.-Y. D., van Swaay, D., deMello, A., Anderson, J. L. R. & Mann, S. In vitro gene expression within membrane-free coacervate protocells. Chem. Commun. 51, 11429–11432 (2015).
Tang, T.-Y. D. et al. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat. Chem. 6, 527–533 (2014).
Mason, A. F., Buddingh, B. C., Williams, D. S. & van Hest, J. C. M. Hierarchical self-assembly of a copolymer-stabilized coacervate protocell. J. Am. Chem. Soc. 139, 17309–17312 (2017).
Fothergill, J., Li, M., Davis, S. A., Cunningham, J. A. & Mann, S. Nanoparticle-based membrane assembly and silicification in coacervate microdroplets as a route to complex colloidosomes. Langmuir 30, 14591–14596 (2014).
Deng, N.-N. & Huck, W. T. S. Microfluidic formation of monodisperse coacervate organelles in liposomes. Angew. Chem. Int. Ed. 56, 9736–9740 (2017).
Long, M. S., Cans, A.-S. & Keating, C. D. Budding and asymmetric protein microcompartmentation in giant vesicles containing two aqueous phases. J. Am. Chem. Soc. 130, 756–762 (2008).
Booth, R., Qiao, Y., Li, M. & Mann, S. Spatial positioning and chemical coupling in coacervate‐in‐proteinosome protocells. Angew. Chem. Int. Ed. 58, 9120–9124 (2019).
Blocher, W. C. & Perry, S. L. Complex coacervate-based materials for biomedicine. WIREs Nanomed. Nanobiotechnol. 9, e1442 (2017).
Chu, H., Gao, J., Chen, C.-W., Huard, J. & Wang, Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc. Natl Acad. Sci. USA 108, 13444–13449 (2011).
Chen, W. C. W. et al. Controlled dual delivery of fibroblast growth factor-2 and interleukin-10 by heparin-based coacervate synergistically enhances ischemic heart repair. Biomaterials 72, 138–151 (2015).
Chu, H., Chen, C.-W., Huard, J. & Wang, Y. The effect of a heparin-based coacervate of fibroblast growth factor-2 on scarring in the infarcted myocardium. Biomaterials 34, 1747–1756 (2013).
Hu, C.-M. J. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Hu, C.-M. J., Fang, R. H., Luk, B. T. & Zhang, L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 8, 933–938 (2013).
Sokol, R. J., Heubi, J. E., Iannaccone, S., Bove, K. E. & Balistreri, W. F. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology 85, 1172–1182 (1983).
Huang, J., Sommers, E. M., Kim-Shapiro, D. B. & King, S. B. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. J. Am. Chem. Soc. 124, 3473–3480 (2002).
Gewaltig, M. T. & Kojda, G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc. Res. 55, 250–260 (2002).
Cohen, R. A. et al. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ. Res. 84, 210–219 (1999).
Wang, C., Trudel, L. J., Wogan, G. N. & Deen, W. M. Thresholds of nitric oxide-mediated toxicity in human lymphoblastoid cells. Chem. Res. Toxicol. 16, 1004–1013 (2003).
Pacher, P., Beckman, J. S. & Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 (2007).
Yang, T., Zelikin, A. N. & Chandrawati, R. Progress and promise of nitric oxide-releasing platforms. Adv. Sci. (Weinh.) 5, 1701043 (2018).
Franchi-Micheli, S. et al. Mechanical stretch reveals different components of endothelial-mediated vascular tone in rat aortic strips. Br. J. Pharmacol. 131, 1355–1362 (2000).
Fleming, I. et al. Isometric contraction induces the Ca2+-independent activation of the endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 96, 1123–1128 (1999).
Acknowledgements
We thank the National Natural Science Foundation of China (21735002, 21778016, 21675047, 21874035) for financial support. The work was partly supported by the BBSRC (BB/P017320/1), the ERC Advanced Grant Scheme (EC-2016-ADG 740235) and BrisSynBio, a BBSRC/EPSRC Synthetic Biology Research Centre (BB/L01386X/1). We thank C. Xu for fruitful discussions, and Rencai Animal Hospital in Changsha for rabbit experiments.
Author information
Authors and Affiliations
Contributions
S.L., K.W., J.L. and S.M. conceived the experiments. S.L., Y.Z., Z.Z. and L.X. performed the experiments. All the authors undertook the data analysis, and S.L., J.L. and S.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Experiment Section, Table 1, Figs. 1–23.
Supplementary Data
Raw data.
Rights and permissions
About this article
Cite this article
Liu, S., Zhang, Y., Li, M. et al. Enzyme-mediated nitric oxide production in vasoactive erythrocyte membrane-enclosed coacervate protocells. Nat. Chem. 12, 1165–1173 (2020). https://doi.org/10.1038/s41557-020-00585-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00585-y
This article is cited by
-
Self-assembly of stabilized droplets from liquid–liquid phase separation for higher-order structures and functions
Communications Chemistry (2024)
-
Artificial cells for in vivo biomedical applications through red blood cell biomimicry
Nature Communications (2024)
-
Superstructural ordering in self-sorting coacervate-based protocell networks
Nature Chemistry (2024)
-
Tuning the viscoelastic properties of peptide coacervates by single amino acid mutations and salt kosmotropicity
Communications Chemistry (2024)
-
Non-interfacial self-assembly of synthetic protocells
Biomaterials Research (2023)