Abstract
Purpose
To investigate the chemopreventive potential of resveratrol, a phytoalexin found in seeds and skin of grapes, berries and peanuts in 7,12 dimethyl benz(a)anthracene (DMBA) induced mouse skin tumorigenesis.
Methods
Topical treatment of resveratrol was given to the animals 1 h prior to DMBA for 28 weeks. At the end of the study period, the skin tumors were dissected out and western blotting was carried out to examine the regulation of proteins involved in anti-tumorigenesis in response to resveratrol.
Results
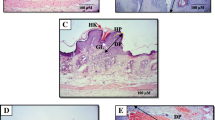
Chemopreventive properties of resveratrol were reflected by delay in onset of tumorigenesis, reduced cumulative number of tumors, and reduction in tumor volume. Results of the western blotting showed that resveratrol treatment increased the DMBA suppressed p53 and Bax while decreased the expression of Bcl-2 and Survivin. Further, resveratrol supplementation resulted in release of cytochrome C, caspases activation, increase in apoptotic protease-activating factor-1 (Apaf-1) as mechanism of apoptosis induction. Resveratrol was also found to inhibit skin tumorigenesis through regulation of Phosphatidylinositol-3-kinase (PI3K)/ and AKT proteins which are implicated in cancer progression because it stimulates proliferation and suppresses apoptosis.
Conclusions
Based on the results we can conclude that resveratrol regulates apoptosis and cell survival in mouse skin tumors as mechanism of chemoprevention hence deserve to be a chemopreventive agent.





Similar content being viewed by others
Change history
15 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11095-020-02973-y
References
D. R. Bickers, and M. Athar. Novel approaches to chemoprevention of skin cancer. J. Dermatol. 27:691–695 (2000).
L. Fremont. Biological effects of resveratrol. Life Sci. 66:663–673 (2000). doi:10.1016/S0024-3205(99)00410-5.
D. K. Das, M. Sato, P. S. Ray, G. Maulik, R. M. Engleman, A. A. Bertelli, and A. Bertelli. Cardio protection of red wine: role of polyphenolic antioxidants. Drugs Exp. Clin. Res. 25:115–120 (1999).
U. R. Pendurthi, F. Meng, N. Mackman, and L.V. Rao. Mechanism of resveratrol mediated suppression of tissue factor gene expression. Thromb. Haemost. 87:155–162 (2002).
S. Banerjee, C. Bueso-Ramos, and B.B. Aggarwal. Suppression of 7, 12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor—Kappa B, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 62:4945–4954 (2002).
M. Jang, L. Cai, O. G. Udeani, K. V. Slowing, C. F. Thomas, C. W. W. Beecher, H. H. S. Fong, N. R. Farnsworth, A. D. Kinghorn, R. G. Mehta, R. C. Moon, and J. M. Pezzuto. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 275:218–220 (1997). doi:10.1126/science.275.5297.218.
M. V. Clement, J. L. Hirpara, and S. Pervaiz. Chemopreventive agent resveratrol, a natural product derived from grapes triggers Cd95 signalling-dependent apoptosis in human tumor cells. Blood. 92:996–1002 (1998).
Y. J. Surh, Y. J. Hurh, J. Y. Kang, E. Lee, G. Kong, and S. J. Lee. Resveratrol,an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 140:1–10 (1999). doi:10.1016/S0304-3835(99)00039-7.
Q. B. She, A. M. Bode, W. Y. Ma, N. Y. Chen, and Z. Dong. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 61:1604–1610 (2001).
N. Ahmad, V. M. Adhami, F. Afaq, D. K. Feyes, and H. Mukhtar. Reveratrol causes WAF-1/p21-mediated G1-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 7:1466–1473 (2001).
R. Joseph, and B. Alfanso. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. 98:10983–10985 (2001). doi:10.1073/pnas.211430998.
N. Kalra, P. Roy, S. Prasad, and Y. Shukla. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 82:348–358 (2008). doi:10.1016/j.lfs.2007.11.006.
A. Singh, and Y. Shukla. Anti tumor activity of diallyl sulphide on polycyclic aromatic hydrocarbon-induced mouse skin carcinogenesis. Cancer Lett. 131:209–214 (1998). doi:10.1016/S0304-3835(98)00152-9.
I. A. Siddiqui, V. M. Adhami, F. Afaq, N. Ahmad, and H. Mukhtar. Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J. Cell Biochem. 91:232–242 (2004). doi:10.1002/jcb.10737.
D. Johnson, and H. Lardy. In R. W. Estabrook, and M. E. Pullman (eds.), Methods in Enzymology, Oxidation and Phosphorylation, Vol. X, Academic, New York, 1967, pp. 94–96.
A. Arora, I. A. Siddiqui, and Y. Shukla. Modulation of p53 in 7,12-dimethylbenz[a] benzanthracene-induced skin tumors by diallyl sulphide in Swiss albino mice. Mol. Cancer Ther. 11:1459–1466 (2004).
O. H. Lowry, N. K. Rosenbrough, and A. L. Farr. Protein measurement with folin phenol reagent. J. Biol. Chem. 193:265–275 (1951).
S. W. Lowe, H. E. Ruley, T. Jacks, and D. E. Housman. p53-dependent apoptosis modulates the cytotoxicity of anticancer drugs. Cell. 74:957–967 (1993). doi:10.1016/0092-8674(93)90719-7.
A. R. Clarke, S. Gledhill, M. L. Hooper, C. C. Bird, and A. H. Wyllie. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene. 9:1767–1773 (1994).
G. J. Kapadia, M. A. Azuine, H. Tokuda, M. Takasaki, T. Mukainaka, T. Konoshima, and H. Nishino. Chemopreventive effectof resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-barr virus early antigen activation assay and the mouse skin two stage carcinogenesis. Pharmacol. Res. 45:499–505 (2002). doi:10.1006/phrs.2002.0992.
V. M. Adhami, F. Afaq, and N. Ahmad. Involvement of the Retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) Cells. Biochem. Biophys. Res. Commun. 288:579–585 (2001). doi:10.1006/bbrc.2001.5819.
G. J. Soleas, L. Grass, P. D. Josephy, D. M. Goldberg, and E. P. Diamandis. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin. Biochem. 35:119–124 (2002). doi:10.1016/S0009-9120(02)00275-8.
Z. D. Fu, Y. Cao, K. F. Wang, S. F. Xu, and R. Han. Chemopreventive effect of resveratrol to cancer. Ai Zheng. 23:869–873 (2004).
J. F Kerr, A. H. Wyllie, and A. R. Currie. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 26:239–257 (1972).
M. Mihara, S. Erster, A. Zaika, O. Petrenko, T. Chittenden, P. Pancake, and U. M. Moll. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 11:77–590 (2003). doi:10.1016/S1097-2765(03)00050-9.
H. J. Harn, L. I. Ho, C. A. Liu, and G. C. Liu. Down regulation of bcl-2 by p53 in nasopharyngeal carcinoma and lack of detection of its specific t (14;18) chromosomal translocation in fixed tissues. Histopathology. 28:317–323 (1996). doi:10.1046/j.1365-2559.1996.d01-431.x.
D. M. Hockenbery, C. D. Giedt, J. W. O’Neill, and M. K. Manion. Mitochondria and apoptosis: new therapeutic targets. Adv. Cancer Res. 85:203–242 (2002). doi:10.1016/S0065-230X(02)85007-2.
L. MacCarthy-Morrogh, A. Mouzakiti, P. Townsend, and M. Brimmell. Bcl-2-related proteins and cancer. Biochem. Soc. Trans. 27:785–789 (1999).
G. S. Salomons, H. J. Brady, M. Verwijs-Janssen, and J. D. Van Den Berg. The Bax:Bcl-2 ratio modulates the response to dexamethasone in leukaemic cells and is highly variable in childhood acute leukaemia. Int. J. Cancer. 71:959–965 (1997). doi:10.1002/(SICI)1097-0215(19970611)71:6<959::AID-IJC9>3.0.CO;2-X.
G. M. Cohen. Caspases: the executioners of apoptosis. Biochem. J. 326:1–16 (1997).
S. Bursztajn, J. J. Feng, S. A. Berman, and A. R. Nanda. Poly (ADP-ribose) polymerase induction is an early signal of apoptosis in human neuroblastoma. Brain Res. Mol. Brain Res. 76:363–376 (2000). doi:10.1016/S0169-328X(00)00026-7.
U. Kolthur-Seetharam, F. Dantzer, M. W. McBurney, G. de Murcia, and P. Sassone-Corsi. Control of AIF mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell cycle. 5:873–877 (2006).
D. C. Altieri. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 22:8581–8589 (2003). doi:10.1038/sj.onc.1207113.
Q. L. Deveraux, and J. C. Reed. IAP family proteins—suppressors of apoptosis. Genes Dev. 13:239–252 (1999). doi:10.1101/gad.13.3.239.
M. Castedo, J. L. Perfettini, T. Roumier, K. Andreau, R. Medema, and G. Kroemer. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 23:2825–2837 (2004). doi:10.1038/sj.onc.1207528.
B. B. Aggarwal, A. Bhardwaj, R. S. Aggarwal, N. P. Seeram, S. Shishodia, and Y. Takada. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical trials. Anticancer Res. 24:2783–2840 (2004).
T. O. Chan, S. E. Rittenhouse, and P. N. Tsichlis. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68:965–1041 (1999). doi:10.1146/annurev.biochem.68.1.965.
R. Srivastava, A. Ratheesh, R. K. Gude, K. V. Rao, D. Panda, and G. Subrahmanyam. Resveratrol inhibits type II phosphatidylinositol turnover. Biochem. Pharmacol. 70:1048–1045 (2005). doi:10.1016/j.bcp.2005.07.003.
T. M. Poolman, L. L. Ng, P. B. Farmer, and M. M. Manson. Inhibition of the respiratory burst by resveratrol in human monocytes: correlation with inhibition of PI3K signalling. Free Radic. Biol. Med. 39:118–132 (2005). doi:10.1016/j.freeradbiomed.2005.02.036.
O. Rachid, and M. Alkhalaf. Resveratrol regulation of PI3K-AKT signaling pathway genes in MDA-MB-231 breast cancer cells. Cancer Genomics Proteomic. 3:383–388 (2006).
Y. Lu, H. Wang, and G. B. Mills. Targeting PI3K-AKT pathway for cancer therapy. Rev. Clin. Exp. Hematol. 7:205–228 (2003).
L. Asnaghi, A. Calastretti, A. Bevilacqua, I. D’Agnano, G. Gatti, G. Canti, D. Delia, S. Capaccioli, and A. Nicolin. Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene. 23:5781–5791 (2004). doi:10.1038/sj.onc.1207698.
Acknowledgement
Authors are thankful to Dr. Ashwani Kumar, Director Indian Institute of Toxicology Research, (Council for Scientific & Industrial Research, India) for his keen interest in the study. Authors are also thankful to Department of Biotechnology (India) for providing fellowship to Ms. Preeti Roy and Indian Council of Medical Research (India) for providing fellowship to Ms. Neetu Kalra and Mr. Sahdeo Prasad.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Roy, P., Kalra, N., Prasad, S. et al. RETRACTED ARTICLE: Chemopreventive Potential of Resveratrol in Mouse Skin Tumors Through Regulation of Mitochondrial and PI3K/AKT Signaling Pathways. Pharm Res 26, 211–217 (2009). https://doi.org/10.1007/s11095-008-9723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9723-z